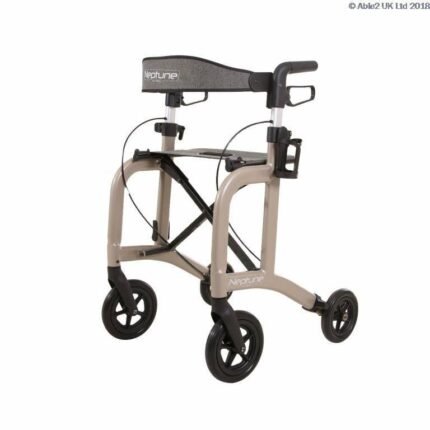
Server HD Lightweight 4 wheel Rollator Walker
Heavy Duty Rollator
The Heavy Duty version of the Rehasense Server rollator range. Weighing less than 8 kg, the Server HD re-enforced, extra wide frame can carry 200 kg.
MPN: 6912
Description
Features
FOLDABLE AND EASY TO CARRY
Server HD folds easily and stays that way with a simple locking system that doubles
up as an ergonomically shaped carrying handle.
HEIGHT ADJUSTABLE
Server HD push handles are height adjustable with
10 positions, from 74 cm to 102 cm.
The seat height is 62 cm with an extra wide 55 cm
seat base. User comfort is ensured with an optimal
frame design and ergonomic push handles.
STEADY AND DURABLE
Frame and seat are tested and approved
for maximum user weight of 200 kg.
The brake mechanism is light yet positive.
TPE-covered, unbreakable wheels.
Usability
For outdoor and indoor use.
Warranty:
2-years limited.
Features
Inclusive crutch holder.
Step pedal and safety reflectors all around.
Practical removable textile shopping bag (5 kg max load).
Backrest
Foldable and easy to carry
Server HD folds easily and stays that way with the locking system that doubles up as an ergonomically shaped carrying handle.
Steady and durable
Frame and seat are tested and approved for maximum user weight of 200 kg. The brake mechanism is light yet positive. TPE-covered, unbreakable wheels.
Sizes
The Server HD is available in size Large and Medium
Seat height: 62 cm.
Seat width 55 cm.
Height adjustable push handles
Server HD push handles are height adjustable with 10 positions, from 74 to 102 cm for Large and 7 positions, from 66 to 86 cm for Medium. The seat height is 62 cm for Large and 55 cm for Medium both with an extra wide 55 cm seat base.
User comfort is ensured with an optimal frame design and ergonomic push handles.
Hand grips
Ergonomic shaped hand grips adjustable to the position of the hand. Smooth operating hand brakes
Server HD Comfort
With soft PU tires for great comfort on uneven ground.
Frame material:
Aluminium
ACCESSORIES
Server HD can be fitted with most of the broad range of Rehasense accessories
Server HD is CE-marked and fulfills the requirements of EU directive 93/42 EEC for medical devices.
Tech Info